Originally Posted by
Excargodog
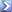
Actually influenza deaths are much lower in the USA. See below. Typically we see less than 10k a year and typically less than 5k. Not to long ago, CDC policy was changed to report influenza with pneumonia deaths together. I'm not going to get into what caused that change in public health policy or why, that is a discussion outside of an airline pilots bulletin board. But, that being said, until there is a reliable mass produced IgG, immunoglobulin G, test to determine actual virus infection rate into the populating we are stuck waiting for a vaccine. I would expect we're 3 months from the IgG test being wildly available. That will help with public transportation.
From April 12, 2009 to April 10, 2010, CDC estimated there were 60.8 million cases (range: 43.3-89.3 million), 274,304 hospitalizations (range: 195,086-402,719), and 12,469 deaths (range: 8868-18,306) in the United States due to the (H1N1)pdm09 virus. These are real numbers with a poor vaccine that year. The trivalent vaccine that year actually completely missed with the quadravalent giving a small response for that H1N1 strain.
News stories and pictures of human patients getting a shot are great for ratings, politicians and stock bumps but we are no where near a vaccine for wide distribution. Many of these studies aren't even giving real vaccines, but just safety studies of delivery platforms. Moderna took a swing and miss already.
Right now J and J does have a candidate and they are taking taking a huge risk by developing and manufacturing concurrently with development. Quite impressive as their risk is high and payoff may never come
Steps for j and j..
1.. refine lead antigen to promote immunological response.
2.. finalize formula and delivery platform
2.. perform carc and mutagenic studies
2.. Perform animal clinical studies
September
3... phase 1 in human clinical study.. small scale
3.. manufacturing and engineering studies
3.. compile data, submissions to FDA
3.. laboratory work
December or January
4.. initiate phase 2a and 2b in human clinical studies... large scale
4... finalize final formula and delivery platform
4.. stability and shipping studies.
4.. manufacturing
4... laboratory work..
4.. compile data and submit to fda.
Maybe next year sometime
5. Large scale phase 3 concurrent with limited approval..
.. this would be how much of the population will start receiving the vaccine ahead of a formal approval.
5.. manufacturing
5... laboratory work..
5.. compile data and submit to fda.
6.. final FDA approval
7.. broad release of the vaccine
7.. collect post approval safety and efficacy data
This is all a very, very, very very advanced timeline. One little hiccup in any step could doom the vaccine and your basically starting from zero.
A few more are close but j and j has the resources. Merk and Sanofi Aventis would be my other bets as they have very good vaccine and manufacturing abilities with very large scientific knowledge banks.
Vaccines are tough to develop. Coronavirus vaccines are difficult, and fingers crossed one is effective soon.