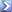View Poll Results: Will you get the vaccine
Yes




282
63.66%
No




161
36.34%
Voters: 443. You may not vote on this poll
Will you get the vaccine?
#101
You’re right. It looks like most in phase 3 have had the second dose, and they’ll continue monitoring for two years: https://www.pfizer.com/news/press-re...vid-19-vaccine
#102
You’re right. It looks like most in phase 3 have had the second dose, and they’ll continue monitoring for two years: https://www.pfizer.com/news/press-re...vid-19-vaccine
Also there is a phase 4: monitoring the real-world deployment for any issues.
But after about six weeks any of the usual immune-induced problems become exceptionally unlikely, and we're well past six weeks for millions of recipients. At this point the only thing they might find in phase 3/4 is going to be quality control or handling issues (we've already seen the later, incorrect doses, vaccine left out of the fridge overnight, etc).
#103
Yes, at this point it's just data collection and paperwork. If there were going to be problems we'd have already seen it by now. I breathed a sigh of relief around Feb 1st.
#104
Also, don't trials for vaccines not approved under an EUA usually involve groups of participants other than those that are healthy adults without any existing health conditions like autoimmune issues or the like?
Not sure I agree that we are out of the woods yet for problems, either - that is why these development periods usually last years and decades rather than months, and even then, historically, they have found problems that did not turn up in trials.
#105
So what is the point of the additional data collection then, if the assumption is that this EUA vaccine is the same as any other normally approved vaccine? is it that the current group of individuals getting vaccinated are akin to test subjects to gather more data points?
Phase 4 always monitors the real-world deployment of pharma, but phase 4 is normally after regular certification. In this case it will inform the regular approval process.
#106
Gets Weekends Off
Joined APC: Oct 2006
Position: Dream Job
Posts: 403
Assumptions don't keep bureaucrats employed, they still have to dot the I's and cross the T's. But for any informed industry observer it's fairly obvious at this point that things are going very well.
Phase 4 always monitors the real-world deployment of pharma, but phase 4 is normally after regular certification. In this case it will inform the regular approval process.
Phase 4 always monitors the real-world deployment of pharma, but phase 4 is normally after regular certification. In this case it will inform the regular approval process.
Sent from my BTV-W09 using Tapatalk
#107
And the real-world results match, Israel has vaccinated millions and observed results identical to the trials (see my post in the Vaccine Progress) thread.
Frankly, we are now beyond pretty much any reasonable doubt about vaccine efficacy or safety for the widely deployed vaccines.
Only things outstanding are...
1) How long does immunity last (natural and vaccine-induced). They have clinically observed a few months, and of course we'll know more as time passes. Historical experience with this sort of virus suggests we'll get at least 1-2 years immunity from vaccines, and it would take a pretty big piece of bad luck to get less than that.
2) Will the bug mutate around the current vaccines? It's possible, but not particularly easy. Even a mutation which results in some reduced vaccine efficacy still probably won't totally nullify the vaccine, and may not be able to overcome herd immunity (once established). Time will tell, but worst case, we modify the vaccines or maybe get period updated boosters. No biggie in the grand scheme, they can stick it in my right arm when they give me the annual flu shot in my left.
Last edited by rickair7777; 02-04-2021 at 01:35 PM.
#108
Gets Weekends Off
Joined APC: Sep 2014
Posts: 1,316
With trials sized at 30,000 people, and control and trial participants intermingled in the same locations statistics tells us the odds of a significant error due to inconsistent exposure between trial groups are astronomically small. They used a large trial group to get results quickly... but one fringe benefit of that is an unusually tight statistical fit on the results (confidence interval). Also HIGHLY telling that both mRNA vaccine trials (run separately in different locations, using technically similar but independently developed and manufactured vaccines) got the same results.
And the real-world results match, Israel has vaccinated millions and observed results identical to the trials (see my post in the Vaccine Progress) thread.
Frankly, we are now beyond pretty much any reasonable doubt about vaccine efficacy or safety for the widely deployed vaccines.
Only things outstanding are...
1) How long does immunity last (natural and vaccine-induced). They have clinically observed a few months, and of course we'll know more as time passes. Historical experience with this sort of virus suggests we'll get at least 1-2 years immunity from vaccines, and it would take a pretty big piece of bad luck to get less than that.
2) Will the bug mutate around the current vaccines? It's possible, but not particularly easy. Even a mutation which results in some reduced vaccine efficacy still probably won't totally nullify the vaccine, and may not be able to overcome herd immunity (once established). Time will tell, but worst case, we modify the vaccines or maybe get period updated boosters. No biggie in the grand scheme, they can stick it in my right arm when they give me the annual flu shot in my left.
And the real-world results match, Israel has vaccinated millions and observed results identical to the trials (see my post in the Vaccine Progress) thread.
Frankly, we are now beyond pretty much any reasonable doubt about vaccine efficacy or safety for the widely deployed vaccines.
Only things outstanding are...
1) How long does immunity last (natural and vaccine-induced). They have clinically observed a few months, and of course we'll know more as time passes. Historical experience with this sort of virus suggests we'll get at least 1-2 years immunity from vaccines, and it would take a pretty big piece of bad luck to get less than that.
2) Will the bug mutate around the current vaccines? It's possible, but not particularly easy. Even a mutation which results in some reduced vaccine efficacy still probably won't totally nullify the vaccine, and may not be able to overcome herd immunity (once established). Time will tell, but worst case, we modify the vaccines or maybe get period updated boosters. No biggie in the grand scheme, they can stick it in my right arm when they give me the annual flu shot in my left.
Last edited by Xtreme87; 02-05-2021 at 04:26 AM.
#109
With trials sized at 30,000 people, and control and trial participants intermingled in the same locations statistics tells us the odds of a significant error due to inconsistent exposure between trial groups are astronomically small. They used a large trial group to get results quickly... but one fringe benefit of that is an unusually tight statistical fit on the results (confidence interval). Also HIGHLY telling that both mRNA vaccine trials (run separately in different locations, using technically similar but independently developed and manufactured vaccines) got the same results.
And the real-world results match, Israel has vaccinated millions and observed results identical to the trials (see my post in the Vaccine Progress) thread.
Frankly, we are now beyond pretty much any reasonable doubt about vaccine efficacy or safety for the widely deployed vaccines.
Only things outstanding are...
1) How long does immunity last (natural and vaccine-induced). They have clinically observed a few months, and of course we'll know more as time passes. Historical experience with this sort of virus suggests we'll get at least 1-2 years immunity from vaccines, and it would take a pretty big piece of bad luck to get less than that.
2) Will the bug mutate around the current vaccines? It's possible, but not particularly easy. Even a mutation which results in some reduced vaccine efficacy still probably won't totally nullify the vaccine, and may not be able to overcome herd immunity (once established). Time will tell, but worst case, we modify the vaccines or maybe get period updated boosters. No biggie in the grand scheme, they can stick it in my right arm when they give me the annual flu shot in my left.
And the real-world results match, Israel has vaccinated millions and observed results identical to the trials (see my post in the Vaccine Progress) thread.
Frankly, we are now beyond pretty much any reasonable doubt about vaccine efficacy or safety for the widely deployed vaccines.
Only things outstanding are...
1) How long does immunity last (natural and vaccine-induced). They have clinically observed a few months, and of course we'll know more as time passes. Historical experience with this sort of virus suggests we'll get at least 1-2 years immunity from vaccines, and it would take a pretty big piece of bad luck to get less than that.
2) Will the bug mutate around the current vaccines? It's possible, but not particularly easy. Even a mutation which results in some reduced vaccine efficacy still probably won't totally nullify the vaccine, and may not be able to overcome herd immunity (once established). Time will tell, but worst case, we modify the vaccines or maybe get period updated boosters. No biggie in the grand scheme, they can stick it in my right arm when they give me the annual flu shot in my left.
#110
I mean this seriously - I have a lot of respect for your opinions and posts, although we clearly have different opinions when it comes to some things - but if this vaccine is just as safe as the other previously approved, then why do we have the lengthier approval process at all? why not just approve all vaccines under Emergency Authority Authorization? I get the taking the financial development risk away from Pharma, and doing bigger phase two trials, but that is clearly not a differentiator here...
Once stage 3 trials are complete, it's in the hands of the government bureaucrats and that normally takes time due to backlog and the general speed of government.
Now there was more hypothetical risk associated with a EUA vaccine on day one back in early Dec. But two months later that risk has been retired. If we were talking some other type of pharma, might be a different story, but vaccine-induced bad reactions are known to occur within about six weeks. That's the nature of the immune system and how it functions. We're now past six weeks with tens (maybe 100?) million people vaccinated.
Also, and this is a biggy, normally both the government and the mfg would like to see how long the vaccine-induced immunity actually lasts before granting certification and going to market. In this case they knew they were good for about six months (based on early trials last summer), and could make a reasonable prediction that it would be good for one year plus. In the covid context, more than enough justification to deploy as EUA. I think that's really what's missing from the EUA data... confirmation of efficacy duration. But they'll have that soon enough anyway.
Like I said other pharmas would be different... if you're evaluating a drug for a chronic condition which patients will be taking for years you'll need to evaluate that drug in use over a longer period of time. But vaccines function on a very short time-line... they trigger an immune response and then get flushed out of your system. That immune response plays out over a relatively short period as well. Once the vaccine is flushed and the immune response has settled down, that's it. Nothing more to see. If the vaccine requires a booster, you do of course need to observe the same process and timeline from the booster.
Thread
Thread Starter
Forum
Replies
Last Post